What is pH? pH in some solutions, common soils
pH is a very common quantity found on product packaging. This is an important indicator used to measure the Acidity or Base of a substance, telling us whether the environment is alkaline or not… In the article below, let’s learn what pH is as well as with Hiep Phat. pH index in some common environments.
What is the pH?

PH is understood as the activity level of Hydrogen ions (H+) in the solution below. affected by an electrolyte constant. The formula for calculating pH is expressed as the negative logarithm of the hydrogen ion concentration running from 0 to 14: pH = -log[H+]
All solutions that exist in liquid form have their own pH and pH affects that liquid for good or bad.< /span>
In the laboratory or in research almost all processes in which water is present require measurement of pH. This includes chemical diagnostics, environmental science water quality testing, and biological environmental experiments.
All living things depend on an appropriate pH level to sustain life.
All people and animals rely on internal mechanisms to maintain a certain pH level.
Uses of pH
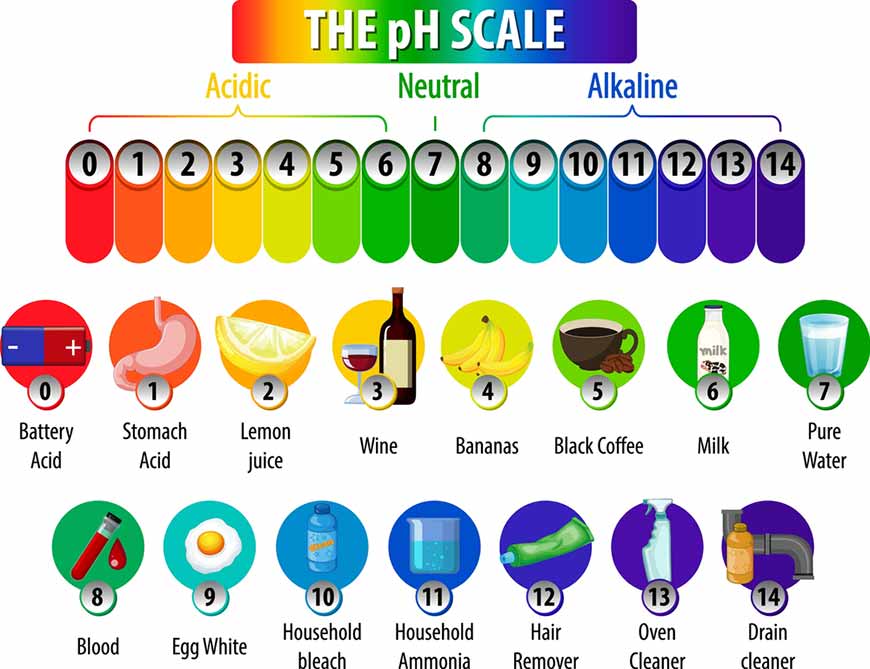
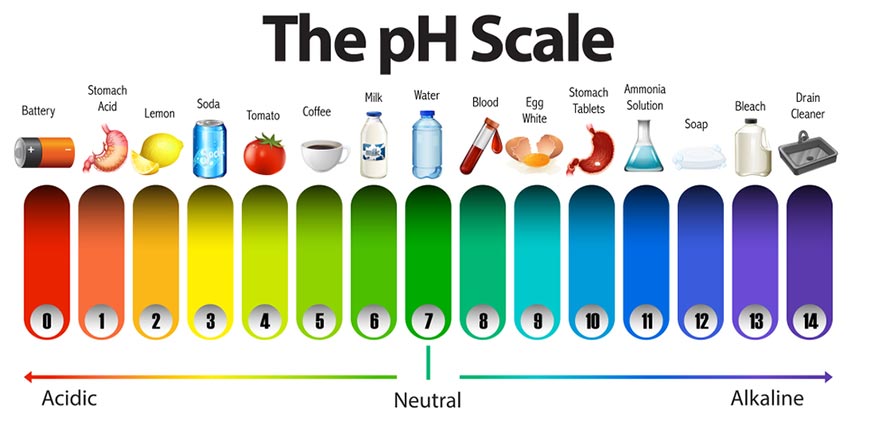
PH is used to distinguish between different types of solutions or the characteristics of each type of solution. By convention, if the amount of H+ ions in the solution is high and active, the solution is acidic (pH < 7). Conversely, if the amount of H+ ions is low, the solution is basic (pH > 7). In case H+ ion is in balance with the amount of Hydroxide (OH-), the solution is neutral, the pH is then approximately 7.
The pH value is an expression of the ratio [H+] to [OH-] (hydroxide ion concentration). Therefore, if [H+] is greater than [OH-], the solution is acidic. Conversely, if [OH‐] is greater than [H+ then the solution is basic.
PH in some solutions, common soils
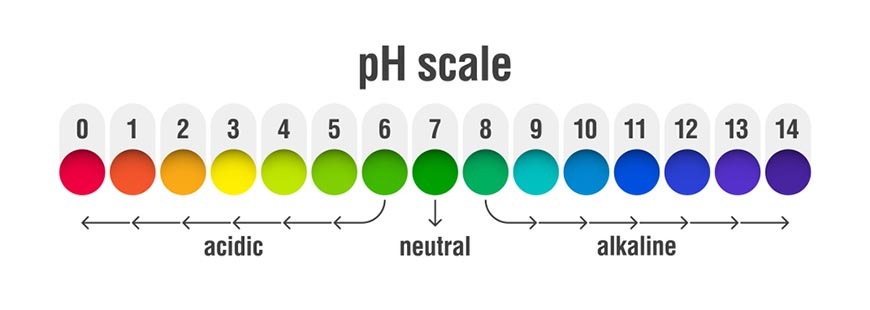
Soil pH
The pH in soil is classified as follows by the United States Department of Agriculture – Natural Resources Conservation Service:
| Name | PH Range |
| Super acid | <3.5 |
| Extremely acidic | 3.5 – 4.4 |
| Very strong acid | 4.5 |
| Strong Acids | 5.1 5.15.5 |
| Moderately acidic | 5,6666.0 |
| Slightly acidic | 6.1 – 6.5 |
| Neutral | 6,6777.3 |
| Slightly alkaline | 7.4 – 7.8 |
| Moderately alkaline | 7.9 – 8.4 |
| Strong alkali | 8.5 |
| Very strong alkali | > 9.0 |
Meanwhile in Vietnam, with many different terrain types and soil types, the pH in soils is regulated as follows:
Alkaline soil is soil with a pH of 7, this is a common soil type in the Southwest region. The feature of this soil is low in nutrients, not suitable for growing agricultural crops.
Neutral soil is soil with a pH of 7, this is a soil suitable for many tropical crops, most notably wet rice. .
Acid soil is soil with a pH less than 7, but the plant is only suitable for soil with a pH between 4 and 7. If pH < 4 is acidic soil.
pH of water
It is well known that water is the most common of all solutions. Water also has many types such as fresh water, salt water, alum water. And the sweetness in water is determined by pH. Each type of water contains its own pH, for example.
The pH of purified water is 7, but note that this pH is only clean water and is treated by filtration methods.
According to standards, the pH of water used for domestic use is 6.0 – 8.5 and that of drinking water is 6.5 – 8.5.
acid pH
Acids have a pH from pH = 0 to ph <7 in the ph scale. Common acidic chemicals commonly encountered in the laboratory are hcl, h2so4.
base pH
Basic or alkaline with pH from 8 to pH 14, common basic aggressive chemicals includes NaOH</a >, KOH…
pH of cleanser
PH value greatly affects the properties and effectiveness of cleansers. Inside the cleanser ingredients will usually include sulfur (S), which exists in the form of acidic compounds. The pH level in the cleanser should be less than 7 and ideally between 6 and 6.5.
urine pH
Every person’s urine has a different pH. The determination of urine concentration helps to check health and detect diseases such as kidney failure, diabetes, kidney stones and especially gastritis caused by hpv virus.
Normally an adult has a urine pH value between 4.6 and >8.
blood pH
The blood flowing through our veins should have a pH value between 7.35 and 7.45. Exceeding this range by as little as one tenth of a pp unit can be fatal.
The importance of pH measurement

The measurement of pH is very important, especially in determining the safety of the products or foods we are dealing with. daily use. Determining the pH value in the foods, cosmetics and necessities we are consuming every day will directly affect the health of each of us.
Our skin and hair have pH values that fluctuate around 5.5, so if we want healthy skin and hair, we should choose cosmetics. have a pH less than 7 to ensure safety.
Fresh food like meat will have to have a pH value of 5.5-6.2. In case the pH is less than 5.3, the meat is probably rancid.
If the pH value of water is too low, it will greatly affect the digestive system and erode tooth enamel or increase metal ions from water containers. If there are organic compounds in the water combined with a pH greater than 8.5, disinfection with chlorine can easily form carcinogenic trihalomethane compounds. … These things will adversely affect health.
If you regularly use water with high pH, it is easy to get diseases related to kidney stones, gallstones,..< /span>
Blood pH is also one of the factors used to determine a person’s health status. In addition, pH is also related to the corrosiveness of equipment, water pipes and water containers.
Signs, how to adjust when the environment has low pH
Signals
Low PH means acidic water, acid in water will cause corrosion of pipes, metal water containers. increase metal ions in water, indirectly affecting human health. Therefore, it is very important to know at what threshold pH value is, especially in determining low pH.
Here are a few signs to identify a low pH:
Most commonly seen are moss green blurs on copper containers, sepia stains on steel objects. A more subtle sign is worn metal objects (signs of acid corrosion).
How to adjust when pH is too low
Use neutral filter
The use of calcium carbonate: If the pH is not too low, filters with the main material Calcite or magnesia can be used to raise the pH. . This type of filter has the ability to filter sediment, so it needs to be washed regularly to avoid clogging. The materials in the filter dissolve slowly and gradually lose. Therefore, it should be regularly checked and supplemented periodically.
Chemical pH adjustment
On a large scale or when the pH is too low, a metering pump is often used to fill soda or a mixture of Soda and Hypochlorite. The adjustment of the pump will be calculated based on the actual, balance between the parameters: pump flow, pH value, concentration of chemical solution to ensure that the pH increases sufficiently. When the water source is contaminated with iron or bacteria, adjusting the concentration of soda and hypochlorite solutions will be more complicated. In some cases, it may be possible to use Potassium to raise the pH, but must be carefully calculated so as not to cause adverse effects on health.
Manual method
For pond water, continuous rain for many days will make the pH in ponds and lakes below 6.5 so people sprinkle lime to pH adjustment.
Also, if you want to adjust drinking water with a low pH value, the simplest way is to use a water purifier that contains filters that create alkalinity, the ability to create electrolyte water and high deoxidation, remove toxins in the water but still retain good minerals for the body.
How to measure pH
Some of the popular methods used to test pH today also compare the advantages and disadvantages so that users can make a choice. for me the most suitable way to test the pH.
Use purple litmus
color-changing purple or neutral litmus paper :
– From initial purple to red to identify solution as acid.
– Turns blue if the solution is alkaline.
Neutral litmus paper contains 10 to 15 different dyes, including azolitmin, leu azolitmin, leuco orcein, and spaniol min.
Advantages:
– This is the simplest, lowest cost method commonly used in labs or education…
– Easy pH determination without much expertise, for fast results.
Cons:
Does not determine the exact pH value but only knows if the solution is acidic, neutral or basic.
Use a pH meter
– Is the most accurate method of determining pH value today. Current pH meters determine to 2 decimal places the pH value.
Advantages:
– Accurately determines the pH of all liquids, beverages, blood or water sources.
– All operations are automatic and display the results on the screen or store the results on the computer.
Cons:
– The cost to buy is quite high
Use a pH meter

This is a widely used method to measure pH, currently there are pH meters divided into 2 types:
– Soil pH meter: is a pen that specializes in measuring the pH of many different types of soil. Determining soil pH helps us to find out what type of soil this is and suitable for which crops.
– Water pH meter: A type of pen that specializes in measuring solution pH, by dipping the probe into the solution. After a few minutes the pen will accurately display the pH in that solution. This is the most commonly used measure of alkalinity in a solution.
Advantage:
– A compact device that can be moved anywhere, easy to store and quick to check.
Cons:
– Not as accurate as a desktop pH meter.
How to store pH measurement methods
If you are conducting wet labs, you will likely need to test pH at some point. Consider cost, accuracy, portability and convenience when choosing a pH test method.
Make sure to store the pH indicator, pH paper or pH meter properly.
Always store test papers in their original containers. Do not expose them to humidity or extreme temperatures. Note the expiration date each time it is used and should follow any accompanying storage instructions.
Before use, test pH meters in known pH solutions (other than water) to ensure they are functioning properly. If they do not give the expected pH value, they should be inspected, repaired, discarded and ordered new to ensure accurate test results.
For long-term storage of handheld devices, remove the batteries to minimize the risk of corrosion, battery leakage, or explosion and damage to the device. Do not store handheld or other measuring equipment in harsh conditions or in a humid environment. Always refer to the manufacturer’s instructions for proper storage.
Hopefully the article has helped you understand the concept of pH as well as how to determine it properly. Customers can also contact Hiep Phat via Hotline: (028) 6287 4765 or Email: sales@thietbihiepphat.com to receive advice on products pH meter and pH meter.

Leave your questions, we will answer them right away



 EN
EN VN
VN